About MSG (UMAMI Seasoning)

Safety of Umami Seasoning “AJI-NO-MOTO®”
What is Umami seasoning “AJI-NO-MOTO®”?
Umami seasoning “AJI-NO-MOTO®” the brand name of the Umami Seasoning product (monosodium glutamate or MSG) which is made from the finest quality crops like sugarcane and tapioca through the natural fermentation process. MSG is a flavour enhancer which has been used effectively for over a century to bring out the best flavour of food.
To know more about MSG, please visit: MSG![]()
To know more about UMAMI Seasoning, please visit: UMAMI![]()
Raw Material Used To Produce Umami Seasoning “AJI-NO-MOTO®”:
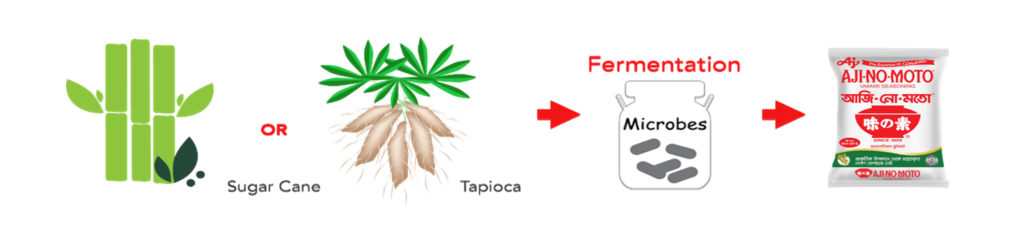
Natural Sources of Glutamate:
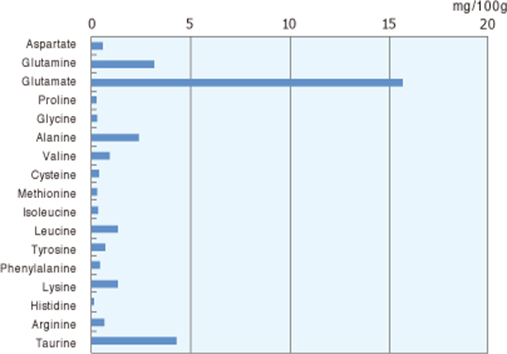
Free Amino Acids in Human Breast Milk:
Truth about Umami Seasoning “AJI-NO-MOTO®“
Safety Assurance
Since 1909, Umami seasoning “AJI-NO-MOTO®” seasoning has been safely used throughout the world and it is also one of the most extensively researched food ingredients with more than hundreds of scientific studies affirming the safety of MSG consumption.
Below are the lists of organizations that have declared the safety of MSG consumption: such as tomatoes and mushrooms, which have high levels of naturally occurring free glutamate.

US FDA
In 1995, the Federation of American Societies for Experimental Biology (FASEB) reaffirmed the safety of MSG for the general population. In its review, commissioned by the FDA, FASEB found no evidence linking MSG to any serious or long-term health effects, which led the FDA to again reaffirm that MSG is a safe food ingredient at normally consumed levels.

FS of Australia New Zealand
Conclusion reached by Foods Standards Australia New Zealand: MSG is safe In 2002, Food Standards Australia New Zealand conducted a review of the safety of MSG. The Chief Scientist concluded that several recent reviews of the scientific evidence have confirmed that MSG is safe for the general public at the levels of use typically found in food.

European Countries
European Communities: ADI not specified In 1991, the European Commission’s Scientific Committee for Food (SCF) also affirmed MSG’s safety. Having reviewed the most advanced and up-to-date research on glutamate, the SCF published a report in 1991 which designated an ‘ADI not specified’ for MSG.

JECFA
Conclusion made by JECFA: ADI not specified JECFA* evaluated the safety of glutamate in 1970, 1973 and 1987. After three safety evaluations, JECFA placed MSG in the safest category, “Acceptable Daily Intake (ADI) not specified” * JECFA is a prestigious scientific advisory body to the World Health Organization (WHO) and the Food and Agricultural Organization (FAO) of the United Nations.

American Academy of Allergy, Asthma & Immunology (AAAAI)
A professional organization with more than 6,700 members in the United States, Canada and 72 other countries with a special interest in the research and treatment of allergic and immunologic diseases (For more info:- http://www.aaaai.org/about-aaaai) AAAAI has concluded that –“There were no reactors in MSG found to be associated with the provocation of Asthma attack” (Read article about Food Allergy: A Practice Parameter) Link:- http://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/food-allergy-2006.pdf

FSSAI
Subjected to Good manufacturing practices (GMP) no defined level prescribed in the FSSAI act, FSSAI Clause No: 3.1.11 Sub Class No: (XXIII): Use of flavor Enhancer (MSG) in following food items is allowed – Noodles seasoning and pasta, meat tenderizer, onion salt, garlic salt, oriental seasoning mix etc. In Table 10 referring to FSSAI in the Seasoning Mix, the culinary powder category dosage of MSG is given as GMP.

